Abstract
Introduction: Haploidentical stem cell transplant (haplo-SCT) with post-transplant cyclophosphamide (PTCY) has become a routine practice in many centers. As for other SCT procedures, post-transplant strategies are developing in order to prevent relapse. Among these options, stimulation of the Vγ9Vδ2 T-cells innate immune effectors is a new possibility. Indeed, this donor population has been associated with improved disease-free survival after SCT. These lymphocytes, which kill tumor cells in a non-MHC restricted manner and do not induce graft-versus-host disease (GVHD) can be generated by stimulation with the bisphosphonate zoledronic acid (ZA), in combination with interleukin-2 (IL-2).
Methods: This French prospective monocentric phase 1 dose-escalating study aimed at demonstrating the possibility to generate Vγ9Vδ2 T-cells in vivo early after haplo-SCT with PTCY. For this purpose, a 3x3 protocol was designed to determine the maximum tolerated dose (MTD) of early administration of increasing low-doses of IL-2 in combination with a fixed dose of ZA. Infusions began on the first Monday following day+15 post-transplant in order to perform IL-2 injections in outpatient mode. ZA (Zometa®, Novartis) was administered once at the recommended dose of 4 mg IV over 15 min, followed, 2 hours later, by the first subcutaneous injection of IL-2 (Proleukin®, Novartis). IL-2 was administered 5 days/week from Monday to Friday for 4 consecutive weeks. Three doses of IL-2 were tested: 2, 4 and 6 million UI/infusion. To avoid ZA side effects, Vitamin D (400 UI/day) and calcium (500mg/day) supplementations were required in all patients (pts) from day+15 until day +45. Main inclusion criteria were: pts aged between 18-70 years old with a hematological disease eligible for haplo-SCT using a Baltimore-based conditioning with fludarabine (Luznik, BBMT, 2008) or clofarabine (Chevallier, Oncotarget, 2018), pts with no HLA-matched sibling or unrelated donor, ECOG status <2, no contra-indication to ZA nor IL-2 and signed informed consent. The MTD was defined by the dose just below that which yielded 2/3 or 2/6 dose limiting toxicities (DLT). The latter included especially severe or reoccurrence of acute GVHD, a potential effect of IL-2. Vγ9Vδ2 T-cells monitoring was performed in multiparameter flow cytometry on blood samples collected before conditioning, on the day of the first ZA and IL-2 injection, then once a week for 4 weeks then at day +70 post-graft. Some pts agreed to be monitored every day during the first 2 weeks of injections. Finally, at least five controls (who did not receive injections but accepted monitoring) were required to compare the effect of both drugs vs not on Vγ9Vδ2 T-cells generation after transplant. The protocol, approved by the Comité de Protection des Personnes of Sud Méditerranée IV (Montpellier) and by French authorities (ANSM) was registered at ClinicalTrials.gov Identifier: NCT03862833.
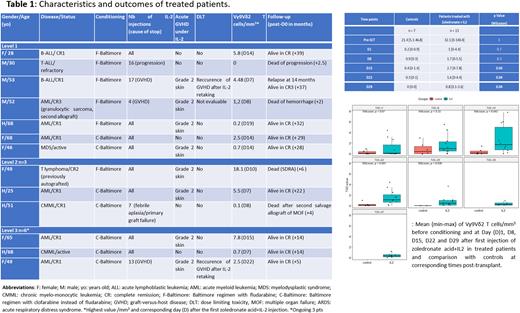
Results: The first pt was included in May 2019. At the time of submission, 13 pts have been treated (level 1 n=7 including 1 not evaluable for DLT, level 2 n=3, level 3 n=3) and 7 controls included. The last three pts are ongoing at level 3 as a DLT has been documented with the first 3 patients. Characteristics and outcomes of participants are described in the Table. First injections occurred at a median of 20 days (range; 18-22) after Day 0. One, 0 and 1 DLT and 4, 2 and 2 acute GVHD (all grade 2) have been documented so far at levels 1, 2 and 3, respectively (Table). Vγ9Vδ2 T cells were clearly detectable in both controls and treated pts before conditioning (Figure). The median highest value (2.5/mm3, range: 0-18.1) was detectable at a median of 12 days after the first ZA and IL-2 injection. A significantly higher proportion of Vγ9Vδ2 T cells was observed at days +15 +22 and +29 in treated pts compared to controls (Figure). Two relapse/progression occurred in treated pts and 9 are still alive in CR (median OS 28 months, range: 5-39). Only 1 out of the 4 deaths was related to the disease.
Conclusion: Early in vivo generation of Vγ9Vδ2 T cells is possible after haplo-SCT with PTCY by using a combination of ZA and repeated IL-2 infusions. The DMT has not been determined so far as the last 3 pts are ongoing. Final results will be presented at the meeting, yet these preliminary data already provide a strong rationale for future phase 2/3 studies with the hope to document lesser post-transplant relapse with this particular adaptive immunotherapy.
Disclosures
Chevallier:Abbvie: Honoraria; Pfizer: Research Funding; Incyte: Research Funding; Jazz Pharmaceuticals: Honoraria; Takeda: Honoraria.
OffLabel Disclosure:
In this study, bisphosphonate zoledronic acid is used in combination with interleukin-2 to generate Vγ9Vδ2 T Cells
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal